Ramiro Gilardino (left) brings 10+ years of experience in health economics, outcomes research, and global health policy. His areas of interest are the sustainability of healthcare systems, access to innovation, and novel elements for assessing the value of healthcare interventions. He also is an independent researcher in global health policy.
Ramiro Gilardino (left) brings 10+ years of experience in health economics, outcomes research, and global health policy. His areas of interest are the sustainability of healthcare systems, access to innovation, and novel elements for assessing the value of healthcare interventions. He also is an independent researcher in global health policy.
Victoria Malek-Pascha (right) gained experience in public and private health sectors and received training across 5 different health systems in 4 continents. She acts as an international consultant in public health and health economics topics. Her interests are focused on the use of health information systems and technologies to improve health systems outreach and access to medicines.
Vaccines are recognised as preventing a wide range of diseases and reducing child mortality. From 2000-2010 they eliminated a half-million deaths from preventable diseases.1 In spite off immunisation being one of the core governmental responsibilities, the effectiveness of new vaccines have typically been reduced by delayed uptake, especially in low to middle-income countries.2
The 2011-2020 Global Vaccine Action Plan increased immunization rates globally, by reducing barriers and inequalities worldwide, thereby helping to meet the Sustainable Development Goal nº3.1,3
Further challenges regarding access and accessibility are still to come.
Currently, more than 200 SARS-CoV-2 vaccine candidates are at different stages of development, nine of which in phase III.4 While uncertainty about the effectiveness, safety, and power of the antibody response exist, further challenges regarding access and accessibility are still to come.
Global initiatives like COVAX may guarantee timely access to SARS-CoV-2 vaccines. However, we recommend considering five critical issues when developing COVID vaccination plans to ensure efficient access and affordability:
1) Clear Regulatory Pathways: While regulatory bodies evaluate the quality and safety of the new vaccines, additional regulatory pathways to address potential emergency approval, as well as outsourced manufacturing to increase the production capacity, should also be considered.
2) Regional and Local Governance: During the pre-approval phase, governing bodies at national or supranational levels should elaborate, discuss, and deploy vaccination rules. One example is the European Union strategy for COVID-19 vaccines, which mandates timely access to vaccines for its population at risk, among other directives.
3) Guarantee Timely Access to Vaccines: Clear vaccination guidelines and pathways that prioritize access for at-risk populations are essential. Engaging public health organizations, clinicians groups (e.g., epidemiologists, infectious disease specialists), and policymakers will be necessary to guarantee timely access.
4) Ensure Accessibility and Affordability: Explore the development of integrated access models to vaccines, including purchasing agreements (i.e. joint purchasing) or managed entry agreements. The involvement of the private sector, including the manufacturers would support both the value chain and the timely access for the population.
5) Analyze potential synergies among funders: Synergistic relationships between global institutions, funding organizations, and donors (i.e, Gates Foundation, The Global Alliance for Vaccine and Immunization), and the private sector, including pharmaceutical manufacturers, may help expedite access to COVID-19 vaccines.
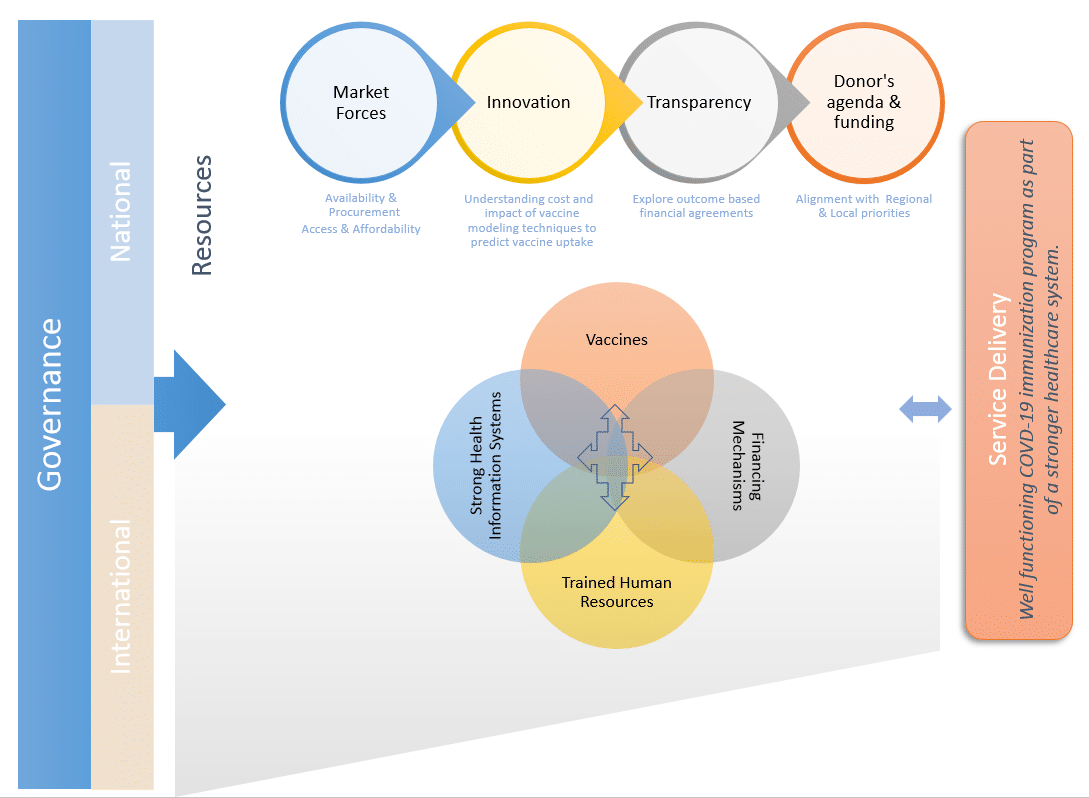
Given the widespread interest in COVID prevention, we anticipate that most individuals will actively request vaccination campaigns. Hence, on the demand side, the societal perspective, and the impact on wealth should be considered when preparing innovative strategies to extend and distribute vaccines equitably (figure – click to enlarge).5 The adoption of appropriate value assessment frameworks that include vaccine-related elements of value (like fear of contagion, impact on productivity, and the burden of disease)6,7 could support decision-makers in resource allocation and tailoring the vaccination strategies.
Finally, we want to emphasize that safe and robust immunization programs are critical to building equitable and resilient health systems.3 Certainly, insufficient access to the SARS-CoV-2 vaccine should not jeopardize it the foundation of such systems. While this piece may not address all concerns regarding the implementation of COVID vaccination programs, we believe it does help generate a more nuanced conversation surrounding vaccine access and affordability during this critical time.
References:
- Global Vaccine Action Plan. Decade of vaccine collaboration. Vaccine. 2013;31 Suppl 2:B5-31. doi:10.1016/j.vaccine.2013.02.015.
- Levine OS, Hajjeh R, Wecker J, et al. A policy framework for accelerating adoption of new vaccines. Hum Vaccin. 2010;6(12):1021-1024. doi:10.4161/hv.6.12.13076.
- World Health Organisation Regional Office for Europe. European Vaccine Action Plan 2015–2020 (2014).; 2017. http://www.euro.who.int/pubrequest. Accessed September 24, 2020.
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Published 2020. Accessed September 24, 2020.
- Bigdeli M, Jacobs B, Tomson G, et al. Access to medicines from a health system perspective. Health Policy Plan. 2013;28(7):692-704. doi:10.1093/heapol/czs108.
- Kamal-Bahl S, Willke RJ, Puckett JT, Doshi JA. The Case For Using Novel Value Elements When Assessing COVID-19 Vaccines And Therapeutics | Health Affairs. Health Affais. https://www.healthaffairs.org/do/10.1377/hblog20200616.451000/full/. Published 2020. Accessed September 24, 2020.
- Brassel, S., Neri, M., O’Neill, P. and Steuten, L. Realising the Value of Vaccines in the UK. OHE Consulting Report. London, UK; 2020. https://www.ohe.org/publications/realising-broader-value-vaccines-uk.
Figure copyright Ramiro Gilardino and Victoria Malek-Pascha, reproduced with permission.
Featured photo by Engin Akyurt at Unsplash